|
|
|
|
|
Mixtures are two or more things that are mixed together but not permanently joined. A good example of a mixture is a salad. There are tomatoes, lettuce, cucumbers, and salad dressing all mixed together. They are mixed up but not joined together. You can separate each of the vegetables from each other, and make piles of lettuce, and piles of tomato. |
|
A cake is a bit different. We make a cake by mixing flour and cocoa and eggs and milk together in a bowl. But then we put it in the oven and bake it. When it is baked, it isn't a mixture - it is a compound. That is because the eggs and the flour and other ingredients are joined together. You can't separate out the eggs and the flour any more! |
|
|
|
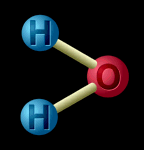
A compound is when two or more kinds of matter are chemically joined. Water, salt, and sugar are examples of compounds. When the particles are joined, the atoms change the way they behave. Water is made up of hydrogen atoms and oxygen atoms joined together. It isn't like hydrogen (a gas) or like oxygen (also a gas) - it is something completely different. And salt isn't like the chlorine (a poisonous gas) or the sodium (a kind of metal) that makes it up, is it? |