|
|
|
|
|
An atom is a really small particle. An atom is so small that it cannot even be seen with a microscope. So scientists had to make predictions about what they really look like. Scientists like John Dalton came up with theories or ideas about atoms. John Dalton thought that atoms were the smallest particles that could exist. He thought that atoms were small little balls packed together. |
|
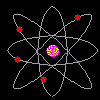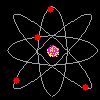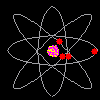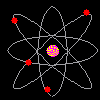
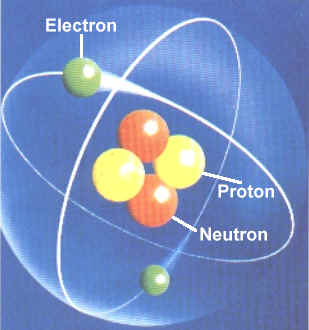
Then scientists found that atoms behaved as though they were made up of tiny particles that had a negative or minus charge. (Remember they can't SEE the atoms - they can only look at the way they behave). These particles were smaller than atoms. They called them electrons, protons and neutrons. This is what an artist thinks an electron might look like. |
 |
 |
Atoms can't be seen and have never been seen. Scientists use models to help understand ideas that cannot be seen directly. The model of the atom was constantly changing. The next model scientists proposed looked like a ball of chocolate chip dough. The cookie dough is the positive part and the chocolate chips are the electrons. |